These antibody-free human primary cells were initiated by elutriation of dispase-dissociated normal human brain cortex tissue without the use of positive selection of antibodies.
Primary Human Brain Microvascular Endothelial Cells (ACBRI 376)
- PRODUCT INFO
- CITATIONS
- TESTS
- RESEARCH
- DOCUMENTATION
Increase Biological Relevance with Human Primary Cells
Our antibody-free primary cells can offer a more biologically relevant cell culture tool for scientists to enhance their research insights. These primary cells were originated using Cell Systems Complete Serum-Free Medium (SF-4Z0-500) and subsequently grown and passaged in Cell Systems Complete Classic Medium (4Z0-500). The cells are cryopreserved in Cell Systems Cell Freezing Medium (4Z0-705).
- Isolated from normal, healthy donor tissue
- Available at Passage 3 (<12 cumulative population doublings)
- Each vial contains approximately 1 x 106 cells
- Supplied as frozen, cryopreserved 1 mL vials or as actively proliferating flask
- Available in reserved lots to enhance consistency in your research program
Each vial of cells is shipped to customers with 1mL of Bac-Off® and .25mL of CultureBoost™.
Cell Profile for ACBRI 376: Surface Markers, Genes & Soluble Molecules (Show More>)
| Cell Marks, Genes & Molecules Tested | Methodologies | Most Cited Studies |
| CD31, CD51, CD54, CD62E, CD105, CD106, Annexin V |
| |
| Ang, FGFa, G-CSF, HGF, IFNg, IL-1a/b, IL-6, IL-8, IL-12, IP-10, Leptin, PIGF, TGFa, TIMP-1/2, TNFa, VEGF |
| |
| CD31, miRNA (miR-181-a, miR-101, miR-30b, miR-27a, miR-21, miR-22, miR-23b, miR-31, miR-103, miR-126, miR-29a, miR-125b, miR-296) |
|
For details on our primary human cell profiles, contact us at customerservice@cell-systems.com
ELEVATE RESEARCH CONSISTENCY
WITH OUR RESERVED LOT PROGRAM
ELEVATE RESEARCH CONSISTENCY
WITH RESERVED LOT PROGRAM
The Reserved Lot Program is designed to assist researchers in securing consistent supplies of human primary cells for their long-term research projects.
How the LOT Program Can Benefit You:
- Reserve a Specific Number of Vials: You can reserve a set number of vials from a designated lot at no cost, ensuring that you have continued access to the same consistent cell lot throughout your research.
- Request New Lots: Our LOT program allows you to request the generation of new lots, with up to 200 vials made specifically for your research needs. While we typically offer 1.00 x 10⁶ cells/vial, we can customize the cell count per vial if necessary to meet your requirements.
RESERVE YOUR LOT TODAY! Email us at customerservice@cell-systems.com or call 425-823-1010.
A Selection of Citations for ACBRI 376 from Scientific Journals
Discover additional research on Google Scholar that utilizes Cell Systems primary cells.
-
"Apoptotic mechanism in human brain microvascular endothelial cells triggered by 4′-iodo-α-pyrrolidinononanophenone: Contribution of decrease in antioxidant properties" Sakai et al. Toxicology Letters, 2021.
-
"Modeling ischemic stroke in a triculture neurovascular unit on-a-chip" Wevers and Nair et al. Fluids and Barriers of the CNS, 2021.
-
"Genetic inhibition of RIPK3 ameliorates functional outcome in controlled cortical impact independent of necroptosis" Wu et al. Cell Death & Disease, 2021.
-
"Triculture Model of In Vitro BBB and its Application to Study BBB-Associated Chemosensitivity and Drug Delivery in Glioblastoma" Seo et al. Advanced Functional Materials, 2021.
- "Human bone marrow-derived mesenchymal stem cells play a role as a vascular pericyte in the reconstruction of human BBB on the angiogenesis microfluidic chip" Kim et al. Biomaterials, 2021
-
"Exosomes Derived from Mesenchyml Stem Cells Ameliorate Oxygen-glucose Deprivation/Reoxygenation-induced Neuronal Injury via Transferring MicroRNA-194 and Targeting Bach1" Li and Zhang et al. Tissue and Cell, 2021.
-
"Paraquat-induced cholesterol biosynthesis proteins dysregulation in human brain microvascular endothelial cells" Tatjana et al. Scientific Reports, 2021.
-
"Blast-induced injury responsive relative gene expression of traumatic brain injury biomarkers in human brain microvascular endothelial cells" Schmitt et al. Brain Research, 2021.
-
"Neuromodulatory effects of SARS-CoV-2 on the blood-brain barrier" Clough et al. Topics in Antiviral Medicine, 2021.
-
"Antiretroviral Tenofovir Induces Senescence-Associated β-Galactosidase Activity in Primary Human Brain Vascular Cells in Multi-Layer Three-Dimensional Co-Culture" Chang et al. Cureus, 2021.
-
"Endothelial Tpl2 regulates vascular barrier function via JNK-mediated degradation of claudin-5 promoting neuroinflammation or tumor metastasis" Nanou et al. Cell Reports, 2021.
-
"Isolation of Primary Human and Rodent Brain Microvascular Endothelial Cells: Culturing, Characterization, and High-Efficiency Transfection" Darbinian et al. Neuronal Cell Culture, 2021.
-
"Increased activation product of complement 4 protein in plasma of individuals with schizophrenia" Kalinowski et al. Translational Psychiatry, 2021.
-
"Exploiting polypharmacology to dissect host kinases and kinase inhibitors that modulate endothelial barrier integrity" Dankwa et al. Cell Chemical Biology, 2021.
- "ALS-causing SOD1 mutants regulate occludin phosphorylation/ubiquitination and endocytic trafficking via the ITCH/Eps15/Rab5 axis" Tang et al. Neurobiology of Disease, 2021.
-
"Latent HIV-Exosomes induce mitochondrial hyperfusion due to loss of phosphorylated dynamin-related protein 1 in brain endothelium" Chandra et al. Molecular Neurobiology, 2021.
-
"ErbB3 is a critical regulator of cytoskeletal dynamics in brain microvascular endothelial cells: Implications for vascular remodeling and blood-brain-barrier modulation" Wu et al. Journal of Cerebral Blood Flow & Metabolism, 2021.
-
"MicroRNA-214 enriched exosomes from human cerebral endothelial cells (hCEC) sensitize hepatocellular carcinoma to anti-cancer drugs" Semaan et al. Oncotarget, 2021.
-
"Enhanced endothelial barrier function by monoclonal antibody activation of vascular endothelial cadherin" Park et al. American Journal of Physiology, 2021.
-
"SARS-COV2 alters blood brain barrier integrity contributing to neuro-inflammation" Reynolds et al. Journal of Neuroimmune Pharmacology, 2021.
-
"Transport of ultrasmall gold nanoparticles (2 nm) across the blood–brain barrier in a six-cell brain spheroid model" Sokolova et al. Scientific Reports, 2020.
-
"Gene expression profiling in human brain microvascular endothelial cells in response to Treponema pallidum subspecies pallidum" Wu et al. SciELO Analytics, 2020.
-
"Endothelial C3a receptor mediates vascular inflammation and BBB permeability during aging" Propson et al. The Journal of Clinical Investigation, 2020.
-
"PBN inhibits a detrimental effect of methamphetamine on brain endothelial cells by alleviating the generation of reactive oxygen species" Hwang et al. Archives of Pharmacal Research, 2020.
-
"Notch1-mediated inflammation is associated with endothelial dysfunction in human brain microvascular endothelial cells upon particulate matter exposure" Park et al. Molecular Toxicology, 2020.
-
"Isolinderalactone suppresses human glioblastoma growth and angiogenic activity in 3D microfluidic chip and in vivo mouse models" Park et al. Cancer Letters, 2020.
-
"Quantitatively relating brain endothelial cell–cell junction phenotype to global and local barrier properties under varied culture conditions via the Junction Analyzer Program" Gray et al. Fluids and Barriers of the CNS, 2020.
-
"Inflammatory Cytokine IL-1β Downregulates Endothelial LRP1 via MicroRNA-mediated Gene Silencing" Hsu et al. Neuroscience, 2020.
-
"Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue organ-on-a-chip platform" Rajan et al. Acta Biomaterialia, 2020.
-
"Towards the development of a human in vitro model of the blood–brain barrier for virus-associated acute encephalopathy: assessment of the time- and concentration-dependent effects of TNF-α on paracellular tightness" Maeda et al. Experimental Brain Research, 2020.
-
"High Glucose and Hypoxia-Mediated Damage to Human Brain Microvessel Endothelial Cells Induces an Altered, Pro-Inflammatory Phenotype in BV-2 Microglia In Vitro" Iannucci et al. Cellular & Molecular Neurobiology, 2020.
-
"In vitro study of transportation of porphyrin immobilized graphene oxide through blood brain barrier" Su et al. Materials Science & Engineering C, 2020.
-
"Altered COVID-19 receptor ACE2 expression in a higher risk group for cerebrovascular disease and ischemic stroke" Choi et al. Biochemical and Biophysical Research Communications, 2020.
-
"Exosome-Mediated Transfer of ACE2 (Angiotensin-Converting Enzyme 2) from Endothelial Progenitor Cells Promotes Survival and Function of Endothelial Cell" Wang et al. Oxidative Medicine and Cellular Longevity, 2020.
-
"Reduced Influence of apoE on Aβ43 Aggregation and Reduced Vascular Aβ43 Toxicity as Compared with Aβ40 and Aβ42." Jäkel et al. Molecular Neurobiology, 2020.
-
"Expression and Functional Relevance of Death-Associated Protein Kinase in Human Drug-Resistant Epileptic Brain: Focusing on the Neurovascular Interface" Williams et al. Molecular Neurobiology, 2019.
-
"FGF20 protected against BBB disruption after traumatic brain injury by activating the AKT/GSK3β pathway to upregulate junction protein expression and inhibiting JNK/NFκB-dependent neuroinflammation" Chen et al. Research Square, 2019.
-
"3D brain angiogenesis model to reconstitute functional human blood–brain barrier in vitro" Lee et al. Biotechnology and Bioengineering, 2019.
-
"Alterations of the Endocannabinoid System in Post-ischemic Endothelial Cells of the Blood Brain Barrier" Thurston (Dissertation) 2019.
-
"Nanomechanical insights: Amyloid beta oligomer-induced senescent brain endothelial cells" Kulkarni et al. BBA-Biomembranes, 2019.
-
"Tumor Cell Mechanosensing During Incorporation into the Brain Microvascular Endothelium" Pranda et al. Cellular and Molecular Bioengineering, 2019.
-
"RAGE and CCR7 mediate the transmigration of Zika-infected monocytes through the blood-brain barrier" Costa de Carvalho et al. Immunobiology, 2019.
-
"Cysteinyl leukotriene receptor type 1 antagonist montelukast protects against injury of blood–brain barrier" Zhou et al. Inflammopharmacology, 2019.
-
"Effects of angiotensin-II on brain endothelial cell permeability via PPARalpha regulation of para- and trans-cellular pathways" Guo et al. Brain Research, 2019.
-
"Effects of lipocalin-2 on brain endothelial adhesion and permeability" Du, Li et al. PLOS One, 2019.
-
"Isolation and characterization of cerebellum-derived stem cells in poststroke human brain" Beppu et al. Stem Cells and Development, 2019.
-
"Oxygen-glucose deprivation/reoxygenation induces human brain microvascular endothelial cell hyperpermeability via VE-cadherin internalization: roles of RhoA/ROCK2" Chen et al. J Molecular Neuroscience, 2019.
-
"Binding Heterogeneity of Plasmodium falciparum to Engineered 3D Brain Microvessels Is Mediated by EPCR and ICAM-1" Bernabeu et al. mBio, 2019.
-
"Ureaplasma species modulate cell adhesion molecules and growth factors in human brain microvascular endothelial cells" Silwedel et al. Cytokine, 2019.
-
"The Membrane-Active Phytopeptide Cycloviolacin O2 Simultaneously Targets HIV-1-infected Cells and Infectious Viral Particles to Potentiate the Efficacy of Antiretroviral Drugs" Gerlach et al. Medicines, 2019.
-
"Application of a Flow-Based Hollow-Fiber Co-Culture System to Study Cellular Influences under Hyperglycemic Conditions" Ebrahim et al. Scientific Reports, 2019.
-
"Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid" Busatto and Vilanilam et al. Cells, 2018.
-
"In Vitro Cell Models of the Human Blood-Brain Barrier: Demonstrating the Beneficial Influence of Shear Stress on Brain Microvascular Endothelial Cell Phenotype" Rochfort and Cummins et al. Blood-Brain Barrier, 2018.
-
"Memantine protects against ischemia/reperfusion‐induced brain endothelial permeability" Liu et al. IUBMB Life, 2018.
-
"Modulation of glucocorticoid receptor in human epileptic endothelial cells impacts drug biotransformation in an in vitro blood–brain barrier model" Ghosh et al. Epilepsia, 2018.
-
"The neurovascular protective effect of alogliptin in murine MCAO model and brain endothelial cells" Hao et al. Biomedicine & Pharmacotherapy, 2018.
-
"HDAC3 inhibition prevents oxygen glucose deprivation/ reoxygenation‐ induced transendothelial permeability by elevating PPARγ activity in vitro" Zhao et al. J Neurochemistry, 2018.
-
"Expression and Functional Relevance of Death-Associated Protein Kinase in Human Drug-Resistant Epileptic Brain: Focusing on the Neurovascular Interface" Williams et al. Molecular Neurobiology, 2018.
-
"Barrier properties and transcriptome expression in human iPSC‐derived models of the blood–brain barrier" Delsing et al. Stem Cells, 2018.
-
"FGF21 Protects the Blood–Brain Barrier by Upregulating PPARγ via FGFR1/β-klotho after Traumatic Brain Injury" Chen et al. J Neurotrauma, 2018.
-
"Recombinant FGF21 Protects Against Blood-Brain Barrier Leakage Through Nrf2 Upregulation in Type 2 Diabetes Mice" Yu et al. Molecular Neurobiology, 2018.
-
"Reactive astrocytic S1P3 signaling modulates the blood–tumor barrier in brain metastases" Gril and Paranjape, et al. Nature Communications, 2018.
-
"High Circulatory Phosphate Level Is Associated with Cerebral Small-Vessel Diseases" Chung et al. Translational Stroke Research, 2018.
-
"Vav3-induced cytoskeletal dynamics contribute to heterotypic properties of endothelial barriers" , et al. Journal of Cell Biology, 2018.
-
"Human Cortex Spheroid with a Functional Blood Brain Barrier for High-Throughput Neurotoxicity Screening and Disease Modeling" Nzou and Wicks et al. Scientific Reports, 2018.
-
"Zika virus crosses an in vitro human blood brain barrier model" Alimonti et al. Fluids and Barriers of the CNS, 2018.
-
"Buprenorphine decreases CCL2‐mediated migration of CD14+CD16+ monocytes" Jaureguiberry‐Bravo et al. J Leukocyte Biology, 2018.
-
"Novel insights into neuroinflammation: bacterial lipopolysaccharide, tumor necrosis factor α, and Ureaplasma species differentially modulate atypical chemokine receptor 3 responses in human brain microvascular endothelial cells" Silwedel et al. J Inflammation, 2018.
-
"Additional increased effects of mannitol-temozolomide combined treatment on blood-brain barrier permeability" Choi et al. BBRC, 2018.
-
"Loading MiR-210 in Endothelial Progenitor Cells Derived Exosomes Boosts Their Beneficial Effects on Hypoxia/Reoxygeneation-Injured Human Endothelial Cells via Protecting Mitochondrial Function" Ma et al. Cellular Physiology and Biochemistry, 2018.
-
"Gintonin, a ginseng-derived exogenous lysophosphatidic acid receptor ligand, enhances blood-brain barrier permeability and brain delivery" Kim et al. Intl Journal of Biological Macromolecules, 2018.
-
"Brain microvascular endothelial cells exhibit lower activation of the alternative complement pathway than glomerular microvscular endothelial cells" Sartain et al. J Biological Chemistry, 2018.
-
"FGF21 Protects the Blood-Brain Barrier by Upregulating PPARγ via FGFR1/β-Klotho Following Traumatic Brain Injury" Chen et al. J Neurotrauma, 2018.
-
"ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway" Zhang et al. J Cellular Molecular Medicine, 2018.
-
"Tick-borne encephalitis virus infects human brain microvascular endothelial cells without compromising blood-brain barrier integrity" Palus et al. Virology, 2017.
-
"Selective inhibition of brain endothelial Rho-kinase-2 provides optimal protection of an in vitro blood-brain barrier from tissue-type plasminogen activator and plasmin" Niego and Lee et al. PLoS ONE, 2017.
-
"α-Klotho expression determines nitric oxide synthesis in response to FGF-23 in human aortic endothelial cells" Chung et al. PLOS One, 2017.
-
"Tetrahydroxy stilbene glucoside ameliorates H2O2-induced human brain microvascular endothelial cell dysfunction in vitro by inhibiting oxidative stress and inflammatory responses" Jiang et al. Molecular Medicine Reports, 2017.
-
"Overexpression of pregnane X and glucocorticoid receptors and the regulation of cytochrome P450 in human epileptic brain endothelial cells" Ghosh et al. Epilepsia, 2017.
-
"Human Endothelial Cell Collection from the Middle Cerebral Artery in Acute Ischemic Stroke" Sheth et al. J Stroke Cerebrovascular Disease, 2017.
-
"Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells" Qian et al. Science Advances, 2017.
-
"Long noncoding RNA MALAT1 inhibits apoptosis induced by oxygen-glucose deprivation and reoxygenation in human brain microvascular endothelial cells" Xin and Jiang, Experimental and Therapeutic Medicine, 2017.
-
"VEGFR2 alteration in Alzheimer’s disease" Cho et al. Scientific Reports, 2017.
Standard Tests
| TESTS | RESULTS |
| HIV Serologic Test (donor level HIV AB EIA) | Negative |
| HIV PCR Test (frozen cell pool by CLIA Licensed Clinical Lab) | Negative |
| Hepatitis B (HBV) and Hepatitis C (HCV) PCR Test (at frozen cell level) | Negative |
Retail Production (P3)Tests
| TESTS | RESULTS |
| Bacterial Sterility (culture method) by independent lab | Pass |
| Fungal Sterility (culture method) by independent lab | Pass |
| Mycoplasma Sterility (culture method) by independent lab | Pass |
Cell Markers and Functional Tests
| MARKER | RESULTS |
| CD31 | > 98% positive by immunofluorescence |
| VWF / Factor VIII | > 95% positive by immunofluorescence |
| Cytoplasmic uptake of Di-I-Ac-LDL | > 95% positive by immunofluorescence |
Immunofluorescence Imagery and Characterization in Peer-Reviewed Literature

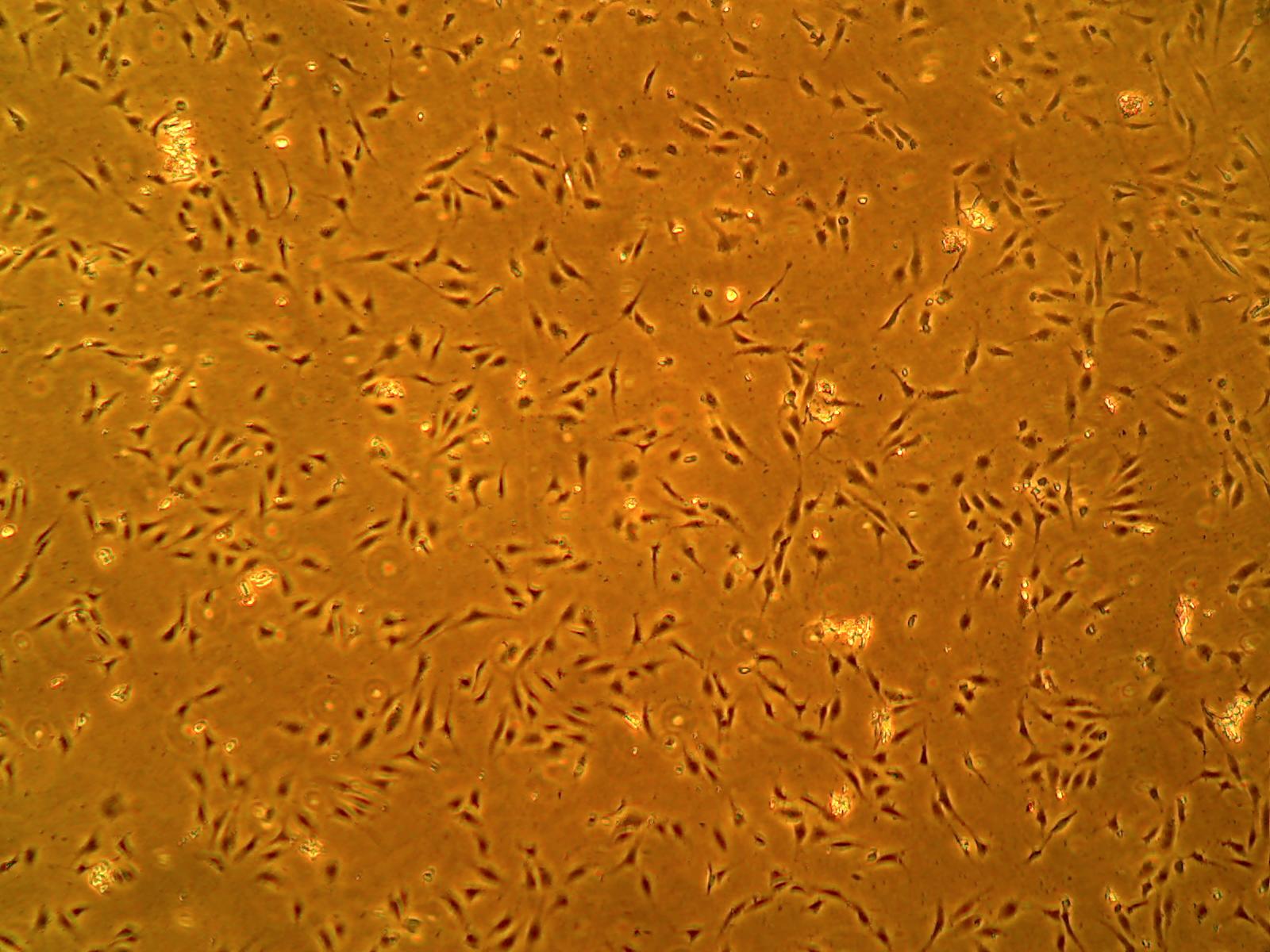
ACBRI 376 cells express endothelial cell markers including CD31 and von Willebrand Factor. They also take up DiI-Ac-LDL in an endothelial cell functional assay.
ACBRI 376 cells express ZO-1 indicating the formation of tight junctions.

Modified with permission from Storm et al EMBO Molecular Medicine (2019) 11:e9164.
Figure S1. Characterisation of HBMEC (Cell Systems ACBRI 376) at number of passages, as measured by flow cytometry. Unlabelled cells are depicted in grey. Blue = passage 5, light green = passage 7 (only in B), red = passage 8, dark green = passage 9. A, Uptake of DiI-labelled acetylated low density lipoprotein. B, Levels of CD31 expression. C, Upregulation of ICAM-1 by 10ng/ml TNF; dotted lines represent unstimulated labelled cells and the continuous lines represent the TNF-stimulated cells. D, Levels of EPCR expression on TNF-stimulated HBMEC. E, Levels of CD36 expression on TNF-stimulated HBMEC.
Reprinted with permission from Smits and Mir et al. PLoS ONE (2011) 6:e16282. Figure 5. Inhibition of EZH2 reduces endothelial tubule formation in vitro. (A) HBMVECs (ACBRI 376) were cultured on Matrigel coated plates in EBM, EGM, or EBM supplemented with U87-GFP cells. Scale bar = 450 µm. Inhibition of EZH2 in HBMVECs, either by transfection with pre-miR-101, EZH2 siRNA, or treatment with DZNep significantly reduced tubule formation as compared to control cells. Tubule formation was assessed as tubule length and branching. (B) Quantitation of tubule length and branching using ImageJ software. (n = 3) Error bars indicate s.d. *p<0.05, ***p<0.001, t test.
Reprinted with permission from Smits and Mir et al. PLoS ONE (2011) 6:e16282. Figure 6. Inhibition of EZH2 reduces endothelial migration and invasion in vitro. (A) HBMVEC (ACBRI 376) monolayer cultures were scratched. Images were acquired directly after scratching (t = 0) and 24 h later (t = 24). The migration front is indicated by the dashed lines. Scale bar = 450 µm. Inhibition of EZH2 in HBMVECs, either by transfection with pre-miR-101, EZH2 siRNA or treatment with DZNep significantly reduced migration as compared to control. (B) Quantitation of endothelial cell migration into the scratched area using ImageJ software. (C and D) HBMVECs were transfected with pre-miR-101, EZH2 siRNA, non-related control molecules, or treated with DZNep, incubated on a Transwell system and subsequently analyzed for invasion capability. EZH2 inhibition, either through pre-miR-101, EZH2 siRNA or DZNep, significantly decreased invasion as shown by Hoechst staining. Scale bar = 225 µm. (n = 3) Error bars indicate s.d. *p<0.05, ***p<0.001, t test.
Reprinted with permission from Niego et al. PLoS ONE (2017) 12: e0177332.
Figure 5. Only KD025, but not fasudil, protects brain endothelial cells (ACBRI 376) from rt-PA and plasmin. (A) Small molecule KD025 concentration-dependently blocks permeability changes in primary human brain microvascular endothelial cells (ACBRI 376 BECs; cultured without astrocytes) treated for 6 h with DMSO as control or with rt-PA (25nM) and human plasminogen (plgn; 100nM), with or without KD025 (2 and 20μM). n = 4. (B) Comparison of selective ROCK-2 inhibition by KD025 (20μM) versus non-selective ROCK inhibition by fasudil (HA1077; 2, 20 and 100μM) against rt-PA+plasminogen (25nM+100nM, respectively) in BECs, as assessed 6 h post stimulation. HA1077 displays no protective capacity in BECs. n = 3. Bars represent mean±SEM. **P<0.01 compared to all other groups, ***P<0.001 compared to t-PA+Plgn by one-way ANOVA with Tukey’s post hoc analysis. ## P<0.01 compared to DMSO control and P = 0.06 by two-tailed paired t-test. (C) Representative phase-contrast micrographs (top panels) and double immunofluorescence images of zonula occludens 1 (ZO-1, representing tight junctions (TJs); white) and nuclei (Hoechst; red) (bottom panels) of ACBRI 376 BECs stimulated for 6 h with DMSO (control) or with rt-PA+plasminogen (25nM+100nM, respectively), in the presence or absence of KD025 (20μM). KD025 attenuates morphological changes (arrows) and preserves TJs in endothelial cells. Asterisks depict areas with degraded TJs. Scale bars = 200μm.
COMPANION PRODUCTS:
OPTIMIZE YOUR RESEARCH
WITH HUMAN PRIMARY CELLS
Avoid the obstacles from immortalized cell lines or animal models and gain more pertinent insights into human cell biology.
Cell Systems cells are available for in vitro research purposes only and may not be transferred out of the direct control of the recipient Institution/Agency/Organization. Cell Systems cells may not be genetically altered in any way without prior written permission from Cell Systems. Use of Cell Systems materials (evidenced by placement of any order for product) constitutes knowledge, understanding and binding acceptance of these restrictions on behalf of the recipient Institution/Agency/Organization.
Cell Systems was created to further the knowledge of eukaryotic cell biology through laboratory research, publications and teaching. Cell Systems provides cells and cell culture products to other research entities - public and private.